Outcomes Database
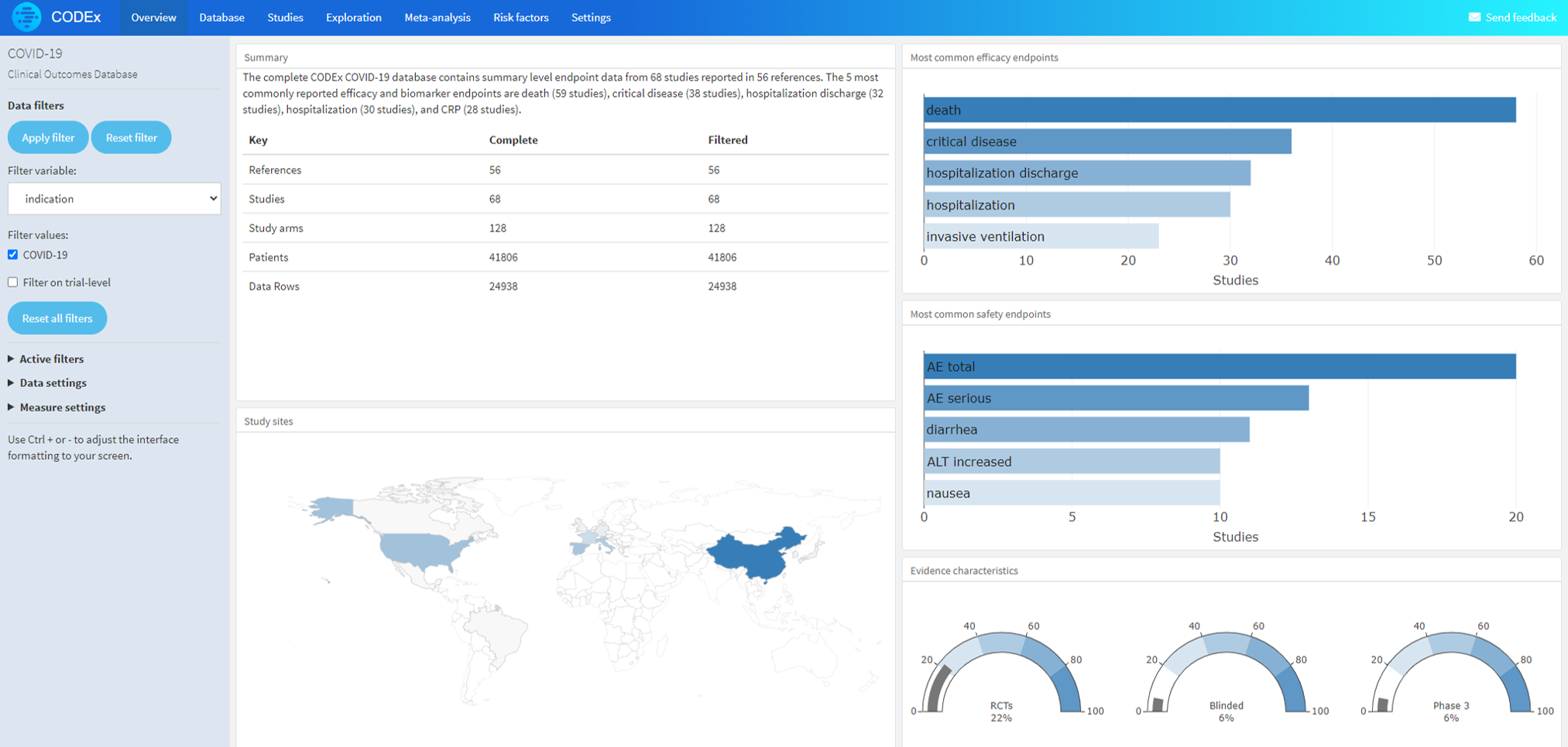
The CODEx COVID-19 database contains summary level endpoint data from 616 studies reported in 610 references. The database will be updated as COVID study results become available.
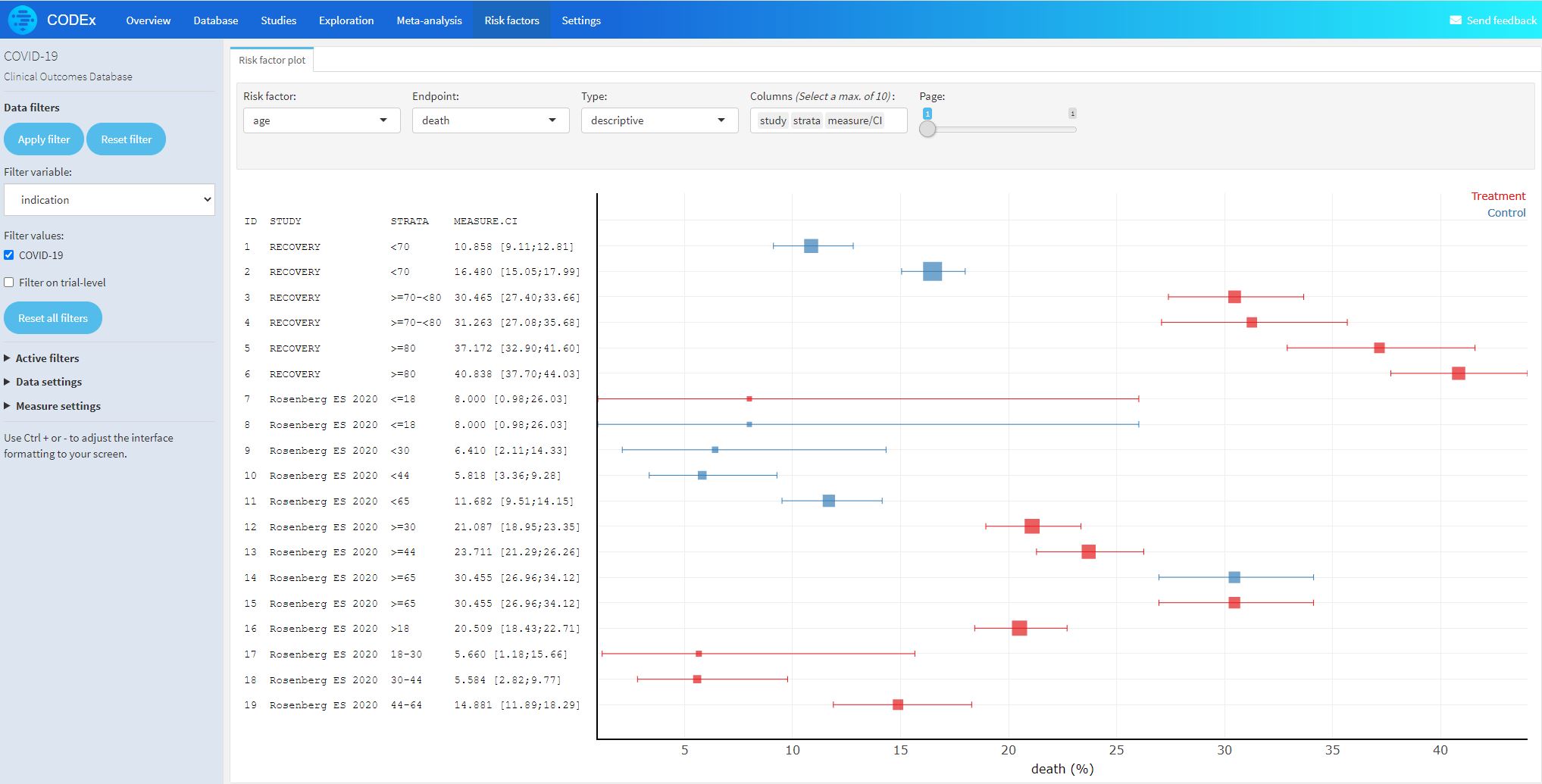
The most common efficacy and biomarker endpoints are death, critical disease, hospitalization, hospitalization discharge, and invasive ventilation.
The Database includes
- All studies (n>10 patients) that evaluate specific treatment options to improve disease outcome
- RCTs, non-randomized controlled, uncontrolled, and retrospective cohorts
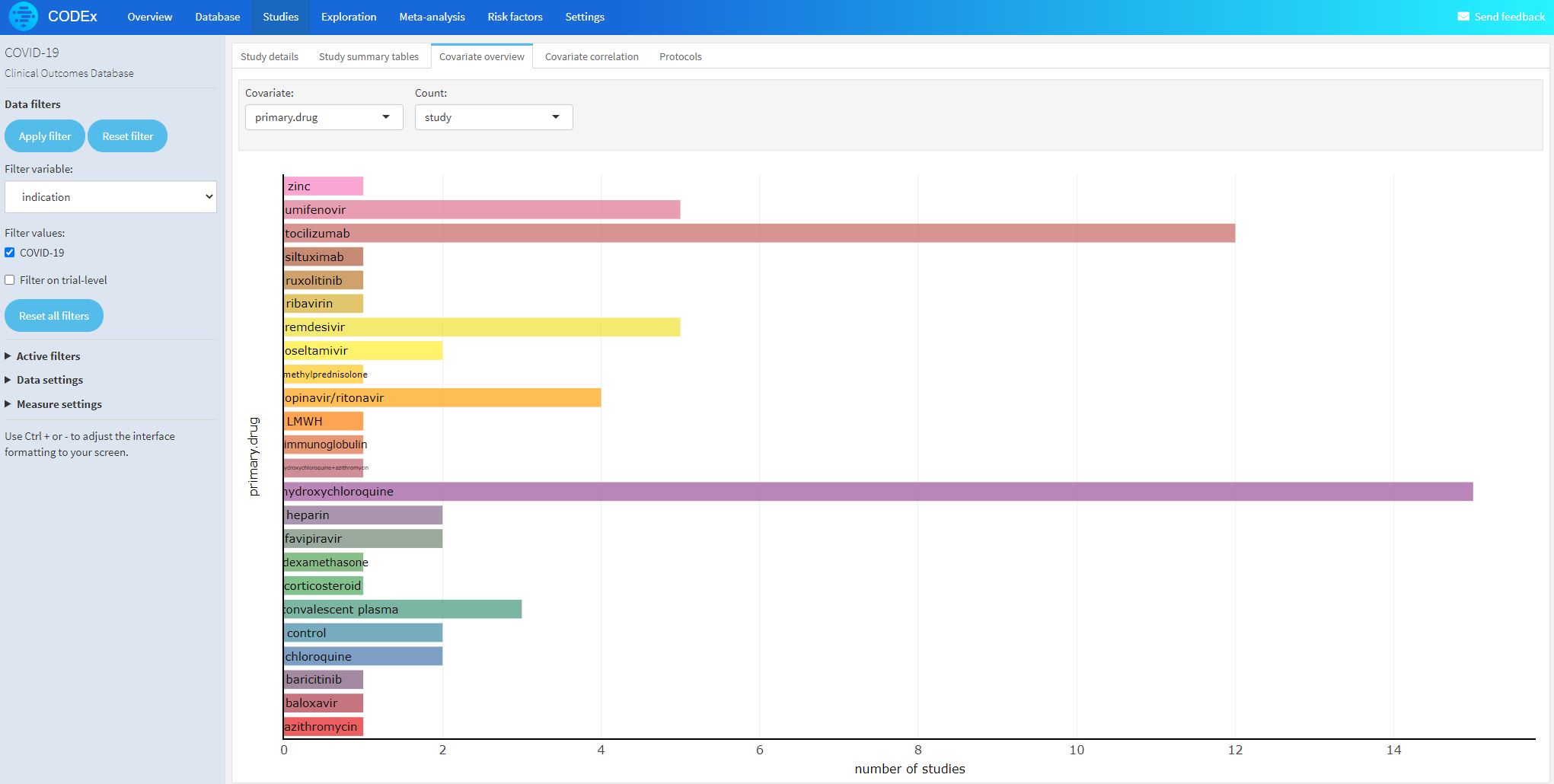
- Anti-malarial, anti-viral, anti IL-6, etc.
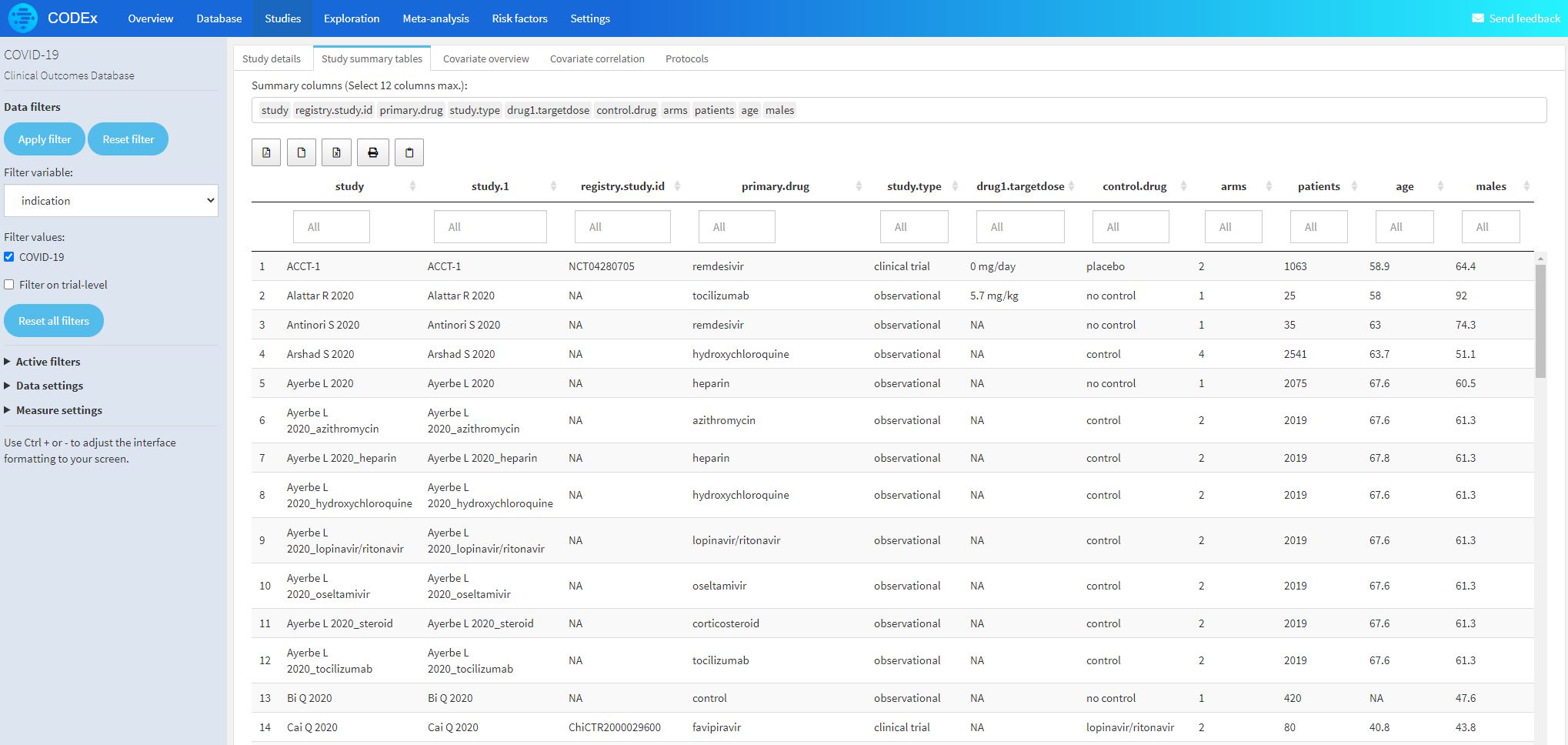
- All patient characteristics, viral load, biomarkers, efficacy, and safety
- Studies (cohorts>100 patients) that evaluate patient characteristics stratified by outcome (independent of treatment)
- Death/no death, progression to severe disease/not, hospitalization/not
- Understand impact of disease and patient characteristics on relative outcome risk
- Can be applied to adjust for impact of patient population differences for meta-analysis of treatment comparisons
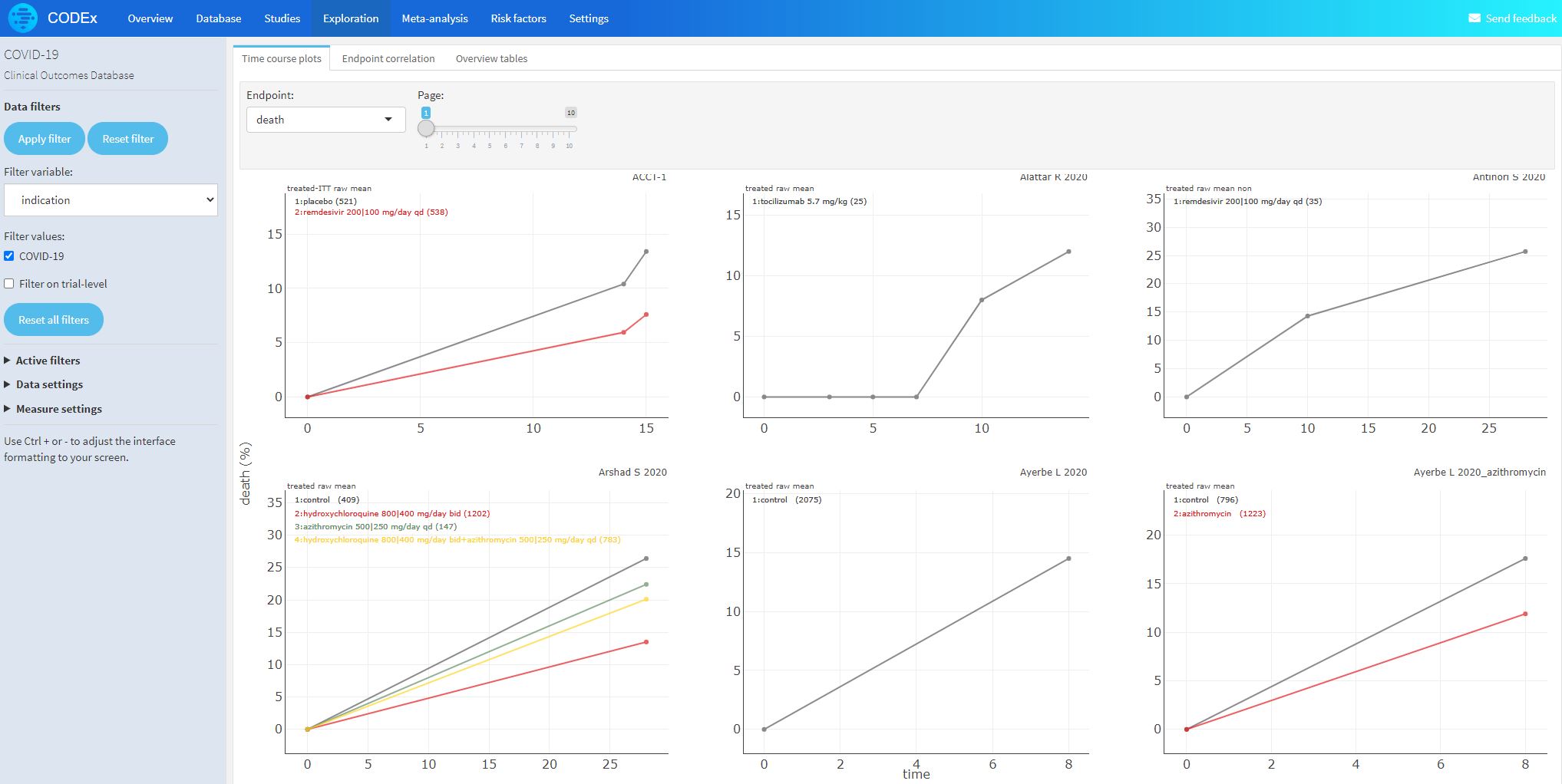
- Studies (n>10 patients) that report the time course of viral load, vital signs, and biomarkers
- Disease model to facilitate understanding of when and how to treat during course of disease
- Characterize longitudinal relationship among viral load, biomarkers, vital signs and outcomes
- Certara’s CODEx platform provides online access, filtering and graphical and tabular summarization of data across studies
- Augmentation of database with standardization of endpoint units, clinical terms, and statistical calculations (i.e. odds-ratios, risk-ratios, mean differences etc.)
- “Automated” pair-wise meta-analysis
To gain access to the COVID-19 Clinical Outcomes Database, please register below. At this time, the database is available to scientists and researchers at academic institutions, non-profit organizations, and biopharmaceutical companies. There is a nominal fee of $500 for 6 months of access to the database, to cover cost of supporting users on the platform. The database is funded by the COVID-19 Therapeutics Accelerator.